The importance of folate (vitamin B9) in the development and function of the nervous system has been well known for many years. Maternal folate deficiency has been linked to neural tube defects such as spina bifida and it is for this reason that women of childbearing age are advised to take a multivitamin with folate.
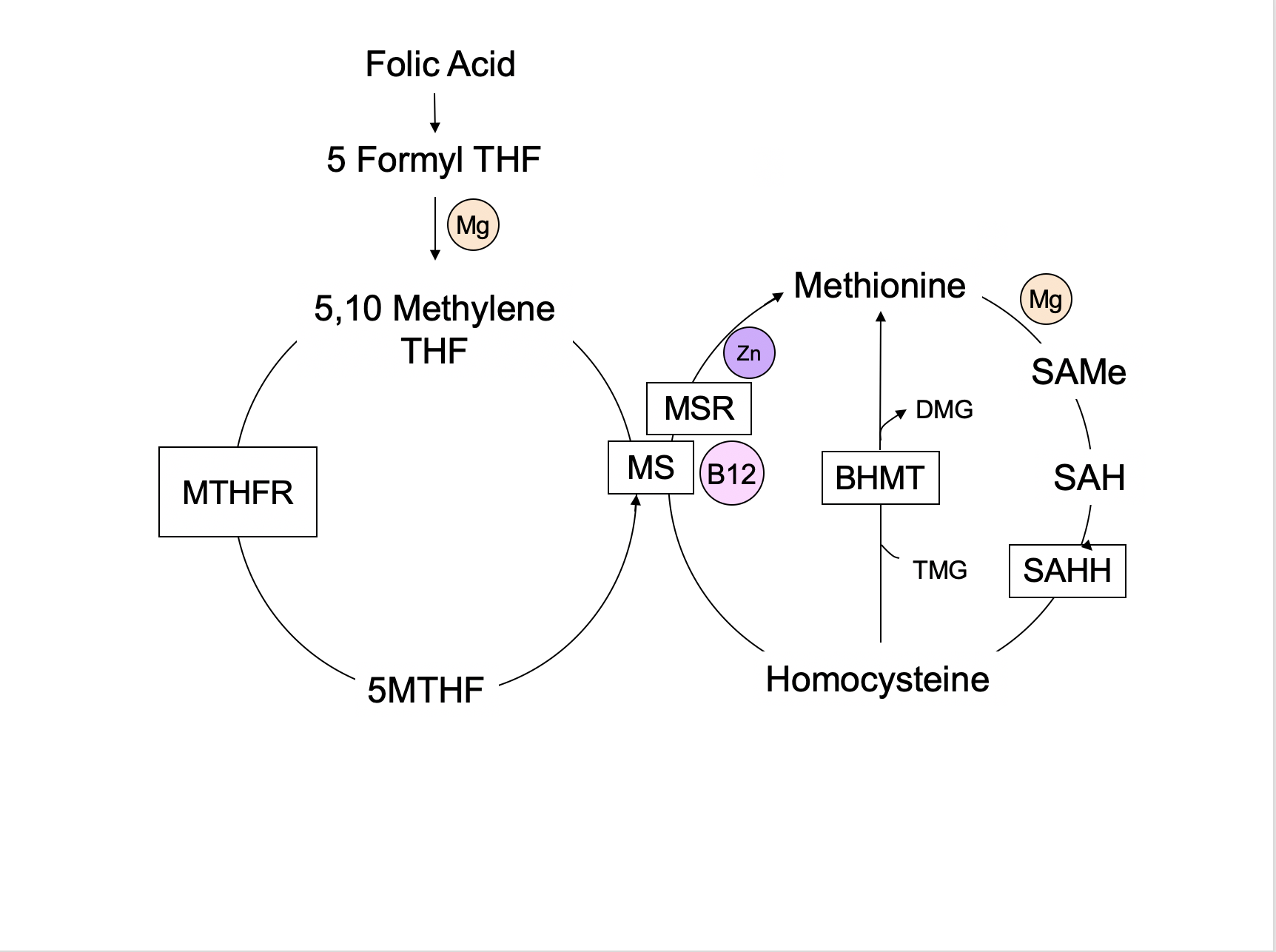
Most foods containing wheat are fortified with a man-made form of folate known as folic acid. While folic acid may be easily converted to the biologically active form of folate found in nature (5-methyltetrahydrofolate, or 5MTHF) by some people, many have difficulty making this conversion due to various genetic mutations. Most notably, mutations in the enzyme methylenetetrahydro-folate (MTHFR) can reduce enzyme activity by as much as 75%, making the ingestion of folic acid through diet and supplements problematic.
Unfortunately, the primary source of folate for most Americans is processed food fortified with folic acid, not the 5MTHF in green vegetables. Folic acid is an oxidized form of folate and of no benefit unless converted to a more bioavailable folate. Folic acid is converted to dihydrofolate and then to tetrahydrofolate (THF). 5MTHF is converted to THF by the MTHFR enzyme, donating its methyl group (a carbon and three hydrogens) to convert homocysteine to methionine via a B12-dependent enzyme. Deficiencies in B12, niacin, folate, or zinc can impair these reactions and lead to widespread metabolic consequences. Elevated homocysteine levels increase the risk of cardiovascular disease, dementia, and many other conditions related to inflammation. This is because glutathione production depends on the availability of 5MTHF. Glutathione is our body’s primary antioxidant. It has the dual role of reducing free radical damage and assisting in the elimination of many toxins.
Folate is also required for the production of every new cell, both during growth and in normal maintenance and repair. It is important in the synthesis of the nucleic acids in our DNA and RNA, making it essential for life. Genes are turned on and off as needed by a process known as methylation, which is folate-dependent. Serotonin, dopamine, and norepinephrine are neurotransmitters that require the conversion of tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2) via the MTHFR enzyme. Disruptions in these pathways, whether caused by nutritional deficiencies, toxins, or genetic mutations, can lead to a lower capacity to make and repair cells, produce neurotransmitters, and eliminate toxins. Blood cells and the cells that line the gastrointestinal tract have a short lifespan and abnormal folate levels are often associated with anemia, low white blood cell counts, and gastrointestinal symptoms. Birth defects, pregnancy losses, and failure to thrive may be seen early in life when folate is critical for growth and development.
In 2002, Dr. Vincent Ramaekers and colleagues reported a series of five patients who experienced developmental regression. These children were initially meeting all developmental milestones, but regressed prior to their first birthdays. Their presenting symptoms included irritability, cognitive decline, ataxia, seizures, and abnormal neurological findings known as pyramidal signs. They also experienced a decrease in head growth (microcephaly). Their blood levels of 5MTHF were normal, yet the level of 5MTHF in their cerebral spinal fluid was low. Ramaekers named this condition cerebral folate deficiency, or CFD. This condition occurs when the immune system produces antibodies that attack or block folate receptor 1 (FR1), the receptor that transports 5MTHF across the blood-brain barrier. This process requires energy in the form of adenosine-5’-triphosphate (ATP). Poor mitochondrial function can further complicate folate transport because ATP production may be impaired.
The causes of CFD are still being delineated. Ramaekers discovered that mutations in FR1 are relatively rare, yet the receptor is clearly not functioning properly. This led to the discovery of anti-folate receptor antibodies in 25 of 28 children with CFD. As suggested earlier, mitochondrial dysfunction can also reduce the transport of folate into the brain, and this has been described both with and without the presence of anti-folate receptor antibodies.
Many children with autism, particularly those who experienced early regression, have been diagnosed with CFD. Mitochondrial disorders are also more prevalent in children with autism than in the general population and mitochondrial dysfunction, the term for decreased mitochondrial function not severe enough to qualify for the diagnosis of a mitochondrial disorder, is very common in children on the autism spectrum. Impaired mitochondrial function is likely a risk factor for the development of CFD and poor methylation is undoubtedly another risk factor.
CFD has also been identified in some children with juvenile rheumatoid arthritis, Down syndrome, and Rett syndrome. It should be noted that MTHFR mutations are common in these populations, as are mitochondrial issues.
Fortunately, folate can also enter the brain through two other mechanisms. The reduced folate carrier (RFC) can transport folinic acid, another form of folate, across the blood-brain barrier and can also escort 5MTHF into neurons once it has entered the cerebral spinal fluid. Folinic acid is also known as 5-formyltetahydrofolate and is sold under the trade name leucovorin. The third mechanism of folate transport across the blood-brain is simple diffusion, which does not require a carrier.
The diagnosis of CFD was originally made by measuring the level of 5MTHF in cerebral spinal fluid, which requires a spinal tap. This procedure is invasive, painful, and not without risk. For this reason, empirical treatment with folinic acid is sometimes initiated when a child’s clinical presentation is consistent with CFD. The identification of anti-folate receptor antibodies is now possible through a commercially available blood test and these results correlate well with CSF findings, making spinal taps unnecessary in some cases.
Testing should be considered in children with autism, Down syndrome, Rett syndrome, seizures, poor mitochondrial function, or developmental regression of any kind. Treatment with folinic acid, often supplemented with 5MTHF and methylcobalamin (B12), can have a profoundly positive effect on the neurological and cognitive development of children with CFD.
The following diagnostic tests are frequently ordered in the workup for CFD:
- Serum folate and B12 levels
- Anti-folate receptor 1 antibody titers (AKA, the FRAT test)
- Thyroid profile
- CBC
- Comprehensive metabolic panel to assess liver and kidney function
- Markers of inflammation such as CRP and sedimentation rate
- Screening tests for autoimmune conditions such as anti-nuclear antibodies (ANA)
- Quantitative plasma amino acids
- Mitochondrial tests: Carnitine profile, acyl carnitine profile, lactate, pyruvate, creatine kinase, coenzyme Q10 level, etc.
- Urinary organic acids
Folinic acid is introduced at a low dose in order to reduce the chance of side effects such as irritability and insomnia. The starting dose is 0.5 to 1 mg/kg per day in divided doses. This is gradually increased to approximately 2 mg/kg per day as tolerated. Vitamin B12 in the form of methylcobalamin, hydroxycobalamin, or adenosylcobalamin should also be prescribed, as it plays a critical role in folate metabolism. Folic acid, the synthetic and inactive form of folate, should be avoided and most patients do best when all gluten and dairy products are eliminated. Dairy intake increases the production of anti-folate receptor antibodies and is not advised.
Additional considerations should include a comprehensive review of any medications, especially antiepileptic medications. Many have a negative impact on folate metabolism and, when possible, should be discontinued or replaced with a safer medication. Screening for nutritional deficiencies, mitochondrial disorders, immune dysregulation, and toxic exposures is also recommended.
CFD is a vastly under-diagnosed condition and many children could enjoy a much higher quality of life if identified and treated. Improvements in language, cognitive function, motor skills, and seizure control are seen in most individuals who undergo a thorough workup and appropriate treatment.
References
- Ramaekers VT, Husler M, Opladen T, Heimann G, Blau N. Psychomotor retardation, spastic paraplegia, cerebellar ataxia and dyskinesia associated with low 5-methyltetrahydrofolate in cerebrospinal fluid: a novel neurometabolic condition responding to folinic acid substitution. Neuropediatrics. 2002 Dec;33(6):301-8.
- Ramaekers VT, Blau N. Cerebral folate deficiency. Dev Med Child Neurol. 2004 Dec;46(12):843-51.
- Ramaekers VT, Rothenberg SP, Sequeira JM, Opladen T, Blau N, Quadros EV, Selhub J. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. NEJM May 2005 12;352(19): 1985-91.
- Ramaekers VT, Hansen SI, Holm J, Opladen T, Senderek J, Husler M, Heimann G, Fowler B, Maiwald R, Blau N. Reduced folate transport to the CNS in female Rett patients. Neurology 2003 Aug 26; 61(4): 506-15.
- Koenig MK, Perez M, Rothenberg S, Butler IJ. Juvenile onset central nervous system folate deficiency and rheumatoid arthritis. J Child Neur Jan 2008; 23(1):106-7. Epub Dec 3 2007.
- Pineda M, Ormazabal A, Lopez-Gallardo E, Nascimento A, Solano A, Herrero MD, Vilaseca MA, Briones P, Ibanez L, Montoya J, Artuch R. Cerebral folate deficiency and leukoencephalopathy caused by a mitochondrial DNA deletion. Ann Neurol Feb 2006;59(2):394-8.
- Ramaekers VT, Weis J, Sequeira JM, Quadros EV, Blau N. Mitochondrial complex I encephalomyopathy and cerebral 5-methyltetrahydrofolate deficiency. Neuropediatrics Aug 2007;38(4):184-7.
- Garcia-Cazoria A, Quadros EV, Nascimento A, Garcia-Silva MT, Briones P, Montoya J, Ormazabal A, Arthuch R, Sequira JM, Blau N, Arenas J, Pineda M, Ramaekers VT. Mitochondrial diseases associated with cerebral folate deficiency. Neurology 2008 Apr 15;70(16): 13600-2.
- Moretti P, Sahoo T, Hyland K, Bottiglieri T, Peters S, del Gaudio D, Roa B, Curry S, Zhu H, Finnell RH, Neul JL, Ramaekers VT, Blau N, Bacino CA, Miller G, Scaglia F. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology 20005 Mar 22;64(6):1088-90.
- Moretti P, Peters SU, Del Gaudio D, Sahoo T, Hyland K, Bottiglieri T, Hopkin RJ, Peach E, Min SH, Goldman D, Roa B, Bacino CA, Scaglia F. Autistic symptoms, developmental regression, mental retardation, epilepsy, and dyskinesias in CNS folate deficiency. J Autism Dev Disord July 2008;38(6):1170-7.
- Rossignol DA, Frye RE. Mitochondrial dysfunction is autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry Jan 2011.
- Ramaekers VT, Blau N, Sequeira JM, Nassogne MC, Quadros EV. Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics Dec 2007;38(6): 276-81.
- Ramaekers VT, Sequeira JM, Artuch R, Blau N, Temudo T, Ormazabal A, Pineda M, Aracil A, Roelens F, Laccone F, Quadros EV. Folate receptor autoantibodies and spinal fluid 5-methyltetrahydrofolate deficiency in Rett syndrome. Neuropediatrics Aug 2007;38(4): 170-83.
- Ramaekers VT, Sequeira JM, Blau N, Quadros EV. A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Dev Med Child Neurol May 2008;50(5):346-52.
- Hansen FJ, Blau N. Cerebral folate deficiency: life-changing supplementation with folinic acid. Mol Genet Metab. 2005 Apr;84(4):371-3.